
Hebrew University researchers achieve breakthrough in naturally immortal cow cells for cultivated beef
Researchers at the Hebrew University of Jerusalem have reported a scientific milestone that could significantly accelerate the development of affordable cultivated beef, revealing for the first time that cow cells can become naturally immortal without genetic modification. The finding challenged longstanding assumptions in cell biology and removed one of the most persistent barriers to producing scalable, regulatory-friendly cultivated meat.
The study, led by Professor Yaakov Nahmias at the Grass Center for Bioengineering at Hebrew University and conducted in collaboration with Believer Meats, was published in Nature Food. The team showed that bovine cells could spontaneously renew themselves indefinitely, a capability previously thought to be limited to smaller animals such as chickens. Until now, cattle cells could only be coaxed into immortalization through gene editing, a process that introduced regulatory and safety concerns for food applications.
Researchers said the discovery opened new pathways for the production of cultivated beef and lamb, which have historically faced higher hurdles than poultry due to the natural resistance of large mammal cells to transformation and renewal.
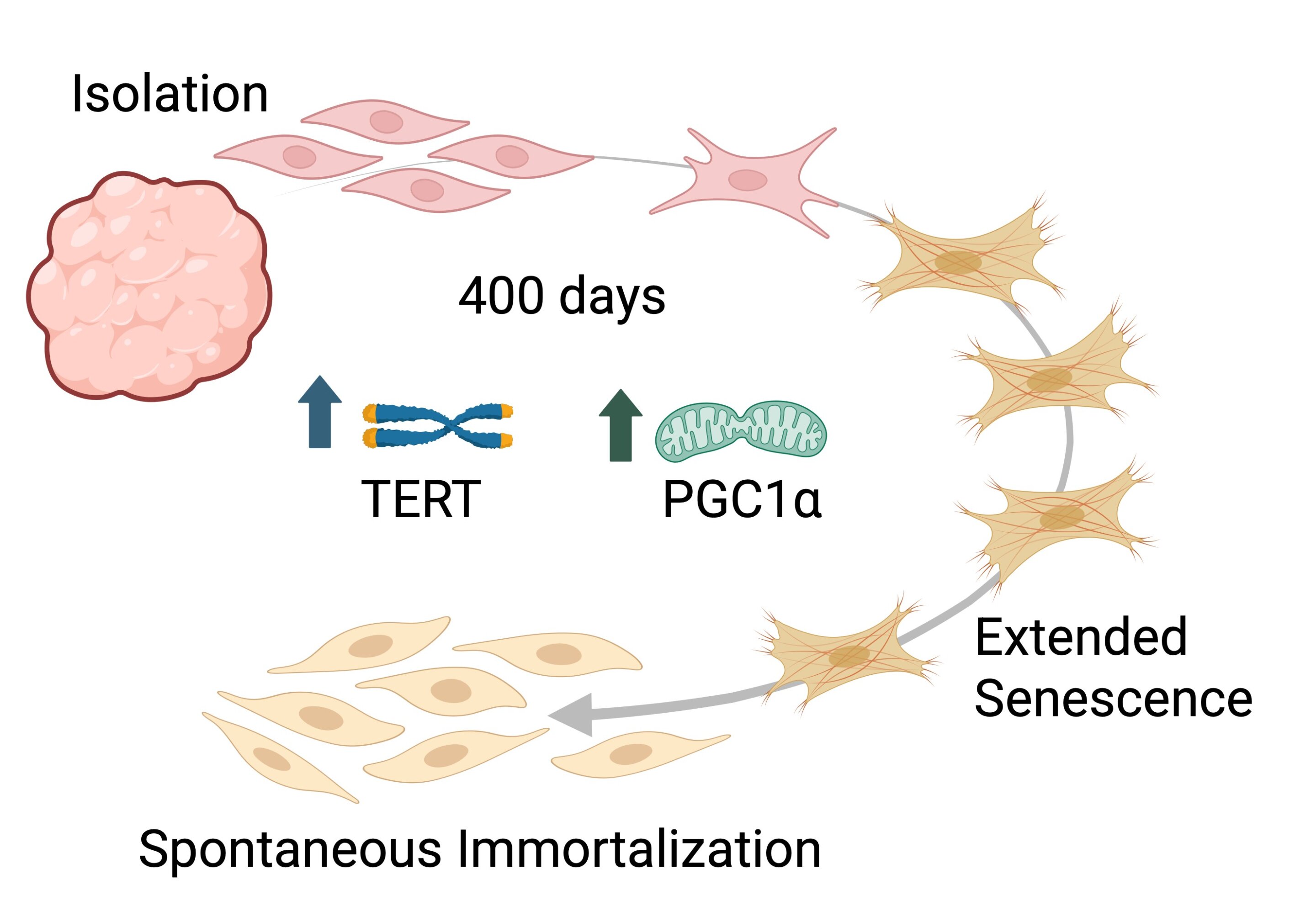
The study followed years of continuous cell culture. Nahmias said his group had first demonstrated spontaneous immortalization in chicken cells several years ago, but the scientific community widely believed that bovine cells were incapable of the same behavior. “We showed chicken cells can immortalize without such interventions a few years ago, but the consensus in the field was that bovine cells could not do the same,” he said. “What worked relatively quickly in chickens became an exhaustive pursuit in bovine cells. We had to continuously culture bovine cells for more than 18 months before the first self-renewing colonies emerged.”
The researchers isolated cells from Holstein and Simmental cows and maintained them in culture for more than 500 days. By day 180 the cells displayed expected signs of aging and senescence, but the team continued the experiment despite the slowdown. After roughly 240 generations, self-renewing bovine cell colonies unexpectedly appeared. Molecular analysis confirmed that the cells had not undergone abnormal growth transformation and retained intact DNA repair mechanisms.
The process was driven by natural activation of telomerase and PGC1α, which allowed the cells to extend chromosomal ends and regenerate mitochondrial function. The team described the result as a controlled, naturally occurring form of immortalization rather than an engineered one, which could ease regulatory concerns about genetically modified food.
Dr Elliot Swartz, Senior Principal Scientist for Cultivated Meat at The Good Food Institute, said the findings were a major step forward for the field. “This work adds valuable new insights to the rapidly expanding knowledge base supporting cultivated meat development. Spontaneous immortalization attempts often fail because researchers simply abandon the process when cell growth slows,” he said. “This study, demonstrating for the first time that bovine cells can be spontaneously immortalized, marks an exciting advance. By detailing the sequence of events that occur during cell line development, it provides a roadmap for non-GM approaches to be used for commercially cultivated meat production across the full range of animal species used in food production.”
The discovery arrived at a critical moment for the cultivated meat sector, which has made advances in poultry and seafood but has struggled to reach similar progress in beef. Beef is among the most resource-intensive forms of agriculture and is linked to deforestation, water use and a substantial share of greenhouse gas emissions. Cultivated beef has long been viewed as a sustainable alternative, but high production costs and technical obstacles have slowed its path to market. Establishing naturally immortal, stable and high-performing cow cell lines is considered foundational to lowering costs and enabling continuous manufacturing.
Nahmias described the breakthrough as the product of persistence. “Months stretched into years, and perseverance replaced certainty. Then, after over 400 silent days, colonies suddenly appeared, a true ‘eureka’ moment that overturned what we thought we knew about bovine cells,” he said.
The research also provided new insight into Peto’s paradox, the observation that large animals develop cancer at surprisingly low rates despite having many more cells. The team said the same natural safeguards that protect large mammals from uncontrolled growth may also make their cells more resistant to renewal, explaining why immortalization in bovine cells required time, adaptation and patience.
The next phase of the work will determine whether naturally immortalized cow cells can be developed into muscle and fat tissues suitable for commercial production. The team also plans to investigate whether similar renewal pathways exist in other mammals, including those used for cultivated lamb and pork.
If successful, the discovery could move the industry closer to cost-competitive cultivated beef, providing a stable, non-GM cell source for large-scale production and reducing reliance on traditional livestock systems.
If you have any questions or would like to get in touch with us, please email info@futureofproteinproduction.com

.png)






